Solvent-driven isomerization of cis,cis-muconic acid for the production of specialty and performance-advantaged cyclic biobased monomers†
Abstract
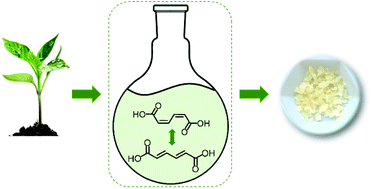
The quest for green plastics calls for new routes to aromatic monomers using biomass as a feedstock. Suitable feedstock molecules and conversion pathways have already been identified for several commodity aromatics through retrosynthetic analysis. However, this approach suffers from some limitations as it targets a single molecule at a time. A more impactful approach would be to target bioprivileged molecules that are intermediates to an array of commodity and specialty chemicals along with novel compounds. Muconic acid (MA) has recently been identified as a bioprivileged intermediate as it gives access to valuable aliphatic and cyclic diacid monomers including terephthalic acid (TPA), 1,4-cyclohexanedicarboxylic acid (CHDA), and novel monounsaturated 1,4-cyclohexenedicarboxylic acids (CH1DA, CH2DA). However, accessing these cyclic monomers from MA requires to first isomerize biologically-produced cis,cis-MA to Diels–Alder active trans,trans-MA. A major impediment in this isomerization is the irreversible ring closing of MA to produce lactones. Herein, we demonstrate a green solvent-mediated isomerization using dimethyl sulfoxide and water. The mechanistic understanding achieved here elucidates the role of low concentrations of water in reducing the acidity of the system, thereby preventing the formation of lactones and improving the selectivity to trans,trans-MA from less than 5% to over 85%. Finally, a Diels–Alder reaction with trans,trans-MA is demonstrated with ethylene. The monounsaturated cyclic diacid obtained through this reaction (CH1DA) can be converted in a single step into TPA and CHDA, or can be directly copolymerized with adipic acid and hexamethylenediamine to tailor the thermal and mechanical properties of conventional Nylon 6,6.


 Please wait while we load your content...
Please wait while we load your content...