Synthesis and polymerisation of α-alkylidene cyclic carbonates from carbon dioxide, epoxides and the primary propargylic alcohol 1,4-butynediol†
Abstract
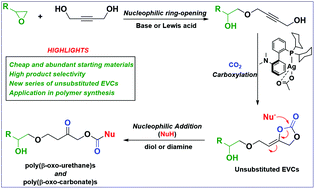
Using the bulk chemical 1,4-butynediol, readily available epoxides and carbon dioxide, a new series of unsubstituted exovinylene carbonates were synthesised. Chemoselective additions of diamines or diols to these cyclic carbonates allow the regiocontrolled synthesis of new functionalised polyurethanes and polycarbonates under mild conditions. This route to polyurethanes avoids the use of toxic isocyanates.


 Please wait while we load your content...
Please wait while we load your content...