Continuous photochemical benzylic bromination using in situ generated Br2: process intensification towards optimal PMI and throughput†
Abstract
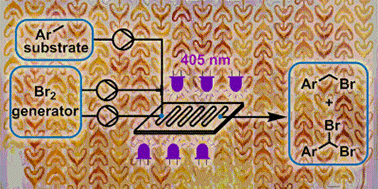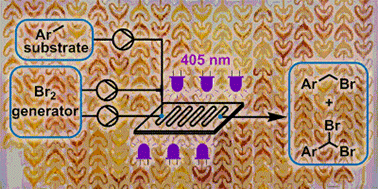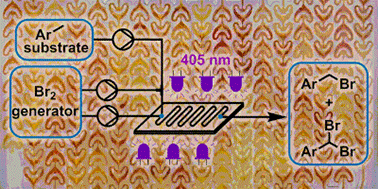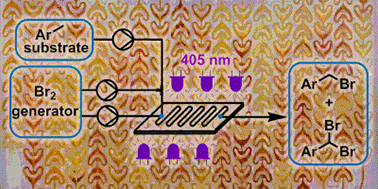
The detailed development of photochemical benzylic brominations using a NaBrO3/HBr bromine generator in continuous flow mode is reported. Optimization of the bromine generator enables highly efficient mass utilization by HBr recycling, coupled with fast interphase transfer within a microstructured photochemical reactor (405 nm LEDs). Intensification of the reaction system, including complete removal of organic solvent, allowed a reduction in PMI from 13.25 to just 4.33. The photochemical transformation achieved exceptionally high throughput, providing complete conversion in residence times as low as 15 s. The organic solvent-free preparation of two pharmaceutically relevant building blocks was demonstrated with outstanding mass efficiency, by monobromination (1.17 kg scale in 230 min, PMI = 3.08) or dibromination (15 g scale in 20 min, PMI = 3.64).


 Please wait while we load your content...
Please wait while we load your content...