Lieb–Oxford bound and pair correlation functions for density-functional methods based on the adiabatic-connection fluctuation-dissipation theorem†
Abstract
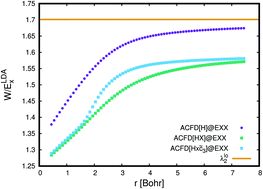
Compliance with the Lieb–Oxford bound for the indirect Coulomb energy and for the exchange–correlation energy is investigated for a number of density-functional methods based on the adiabatic-connection fluctuation-dissipation (ACFD) theorem to treat correlation. Furthermore, the correlation contribution to the pair density resulting from these methods is compared with highly accurate reference values for the helium atom and for the hydrogen molecule at several bond distances. For molecules, the Lieb–Oxford bound is obeyed by all considered methods. For the homogeneous electron gas, it is violated by all methods for low electron densities. The simplest considered ACFD method, the direct random phase approximation (dRPA), violates the Lieb–Oxford bound much earlier than more advanced ACFD methods that, in addition to the simple Hartree kernel, take into account the exchange kernel and an approximate correlation kernel in the calculation of the correlation energy. While the dRPA yields quite poor correlation contributions to the pair density, those from more advanced ACFD methods are physically reasonable but still leave room for improvements, particularly in the case of the stretched hydrogen molecule.
- This article is part of the themed collection: New horizons in density functional theory


 Please wait while we load your content...
Please wait while we load your content...