Enhanced stability of silicon for photoelectrochemical water oxidation through self-healing enabled by an alkaline protective electrolyte†
Abstract
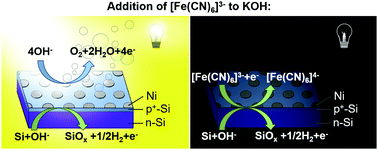
Alkaline electrolytes impede the corrosion of Si photoanodes under positive potentials and/or illumination, due to the formation of a SiOx layer that etches 2–3 orders of magnitude more slowly than Si. Hence during water oxidation under illumination, pinholes in protection layers on Si photoanodes result in the local formation of a protective, stabilizing passive oxide on the Si surface. However, operation under natural diurnal insolation cycles additionally requires protection strategies that minimize the dark corrosive etching rate of Si at pinholes. We show herein that addition of [Fe(CN)6]3− to 1.0 M KOH(aq) results in a self-healing process that extends the lifetime to >280 h of an np+-Si(100) photoanode patterned with an array of Ni catalyst islands operated under simulated day/night cycles. The self-healing [Fe(CN)6]3− additive caused the exposed Si(100) surface to etch >180 times slower than the Si etch rate in 1.0 M KOH(aq) alone. No appreciable difference in etch rate or facet preference was observed between Si(100) and Si(111) surfaces in 1.0 M KOH(aq) with [Fe(CN)6]3−, indicating that the surface conformally oxidized before Si dissolved. The presence of [Fe(CN)6]3− minimally impacted the faradaic efficiency or overpotential of p+-Si/Ni electrodes for the oxygen-evolution reaction.


 Please wait while we load your content...
Please wait while we load your content...