Beyond the concentrated electrolyte: further depleting solvent molecules within a Li+ solvation sheath to stabilize high-energy-density lithium metal batteries†
Abstract
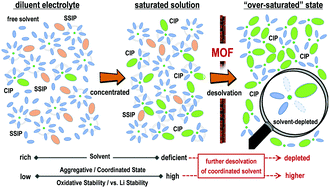
The detrimental decomposition of electrolytes, in particular the dehydrogenation of solvents, would accelerate the degradation of batteries and hinder the development of high-energy-density lithium–metal batteries (LMBs). The purpose of building classic concentrated electrolytes is to decrease the proportion of solvents, so that the solvent-related parasitic reactions can be suppressed. However, accompanied by the reduction of solvents, the electrolyte concentration processes would reach their limits when saturated states are achieved. Herein, beyond the concentrated electrolytes (solvent-definite state), an electrolyte with a more aggregative configuration was obtained after further depleting the solvent molecules within a Li+ solvation sheath. The prepared electrolyte demonstrated a largely expanded electrochemical stability window (enlarged from 4.5 V to 5.4 V vs. Li/Li+), enhanced stability towards high-Ni NCM-811 cathode and thin cathode electrolyte interlayer (CEI) films. Assembled with high-voltage cathodes (NCM-811 and 5.0 V-class LiCoMnO4 (LCMO)) and limited excess lithium metal, high-energy-density LMB full cells (above 630 W h kg−1) with ultra-stable cycling performance were achieved. We expect this electrolyte design strategy to expand the family of electrolytes and remedy the inherent defects of conventional electrolytes for high-energy-density LMBs.


 Please wait while we load your content...
Please wait while we load your content...