Electrochemical CO2 reduction to methane with remarkably high Faradaic efficiency in the presence of a proton permeable membrane†
Abstract
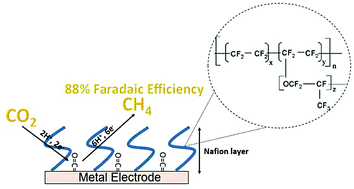
Nafion is a widely used fluoropolymer that is often mixed with electrocatalysts to facilitate proton transport. In contrast to these Nafion-catalyst composites, this work studies electrodes covered by Nafion overlayers for the CO2 reduction reaction. By varying the thickness, substrates, and voltage, we perform a detailed study of the effect of Nafion overlayers on metal and carbon mesh electrodes for CO2 reduction. Depending on the thickness of the Nafion membrane, CO2 reduction occurs at either the polymer–electrolyte interface or electrode–polymer interface. A Nafion overlayer of 15 μm on a Cu electrode enables an extraordinarily high yield of CH4 production (88% Faradaic efficiency) at a low overpotential (540 mV) via the stabilization of metal-bound CO intermediates. To the best of our knowledge, this yield is the highest for electrocatalytic CO2 reduction to CH4 production at room temperature reported.


 Please wait while we load your content...
Please wait while we load your content...