Exploring the artificially induced nonstoichiometric effect of Li2RuO3 as a reactive promoter on electrocatalytic behavior†
Abstract
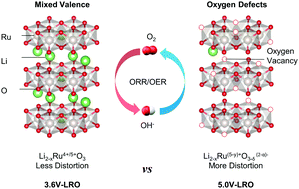
Understanding the fundamental properties, particularly identifying the independent effect of factors that govern catalytic activities, is crucial to design strategies for synthesizing efficient electrocatalysts. Here, we have explored the independent effect of mixed valence and oxygen defects on the catalytic origin by artificially engineering the stoichiometry of Li2RuO3. On the basis of the operando X-ray absorption spectroscopy (XAS) results, we demonstrated that the mixed valence enhanced the redox reversibility of ruthenium ions and the oxygen defects provided more electrocatalytic active sites. Consequently, non-stoichiometric Li2−xRuO3−y exhibited superior performance for the oxygen reduction reaction (ORR), oxygen evolution reaction (OER) and Zn–air batteries in alkaline media compared to state-of-the-art Pt/C and RuO2. Moreover, the applicability of the nonstoichiometric effect in various electrocatalytic reactions was systematically verified. Therefore, artificially induced non-stoichiometry is a feasible strategy to design efficient multifunctional electrocatalysts and this strategy can be applied to general metal oxide electrocatalysts for various electrocatalytic reactions.


 Please wait while we load your content...
Please wait while we load your content...