Towards a generic understanding of oxygen evolution reaction kinetics in polymer electrolyte water electrolysis†
Abstract
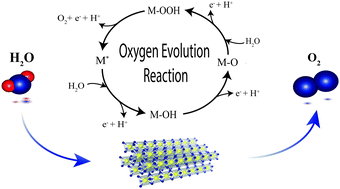
Water electrolysis is a key technology for future energy systems intended for the storage of fluctuating renewables and green industrial product upgrading. Under acidic electrolysis conditions, the oxygen evolution reaction (OER) predominantly causes the overpotential loss, which makes the elucidation of OER kinetics a task of key importance for future catalyst development. Herein, we design a methodology based on vapor-fed polymer electrolyte water electrolysis to fully characterize OER kinetics in the real environment of solid electrolytes and realize the controlled benchmarking of catalysts in the absence of gas passivation commonly observed in liquid-electrolyte half-cell configurations. Vapor-fed cells, allowing for distinct manipulation of water activity, sustain the essential degree of freedom to resolve and determine all four essential kinetic parameters. Thus, this work provides valuable insights into the OER mechanism, isolates the rate-determining step, and experimentally determines the reaction order of the state-of-the-art OER catalyst IrO2/TiO2 with respect to water. Through the combination of gas and liquid fed kinetic analysis, we elucidate the important missing link for formulating the generic governing relation for the OER overpotential and thereby providing a method for benchmarking of catalyst activities under technically representative conditions.


 Please wait while we load your content...
Please wait while we load your content...