Reversible switching between housane and cyclopentanediyl isomers: an isonitrile-catalysed thermal reverse reaction†
Abstract
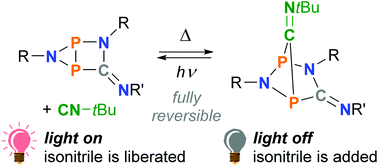
The photo-isomerization of an isolable five-membered singlet biradical based on C, N, and P ([TerNP]2CNDmp, 2a) selectively afforded a closed-shell housane-type isomer (3a) by forming a transannular P–P bond. In the dark, the housane-type species re-isomerized to the biradical, resulting in a fully reversible overall process. In the present study, the influence of tBuNC on the thermal reverse reaction was investigated: the isonitrile acted as a catalyst, thus allowing control over the thermal reaction rate. Moreover, tBuNC also reacted with the biradical to form an adduct species ([TerNP]2CNDmp·CNtBu, 4a), which can be regarded as the resting state of the system. The reactive species 2a and 3a could be re-generated in situ by irradiation with red light. The results of this study extend our understanding of this new class of molecular switches.


 Please wait while we load your content...
Please wait while we load your content...