Bonded- and discreted-Lindqvist hexatungstate-based copper hybrids as heterogeneous catalysts for the one-pot synthesis of 2-phenylquinoxalines via 2-haloanilines with vinyl azides or 3-phenyl-2H-azirines†
Abstract
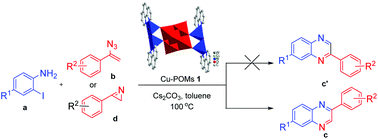
One bonded- and one discreted-Lindqvist hexatungstate-based copper hybrids (Cu-POMs) ([Cu2(O)OH(phen)2]2[W6O19]·6H2O (1) and [Cu2(phen)4Cl] [HW6O19]·2H2O (2) (phen = 1,10-phenanthroline)) were controllably synthesized and routinely characterized. Cu-POMs 1–2 consisted of identical [W6O19] unit and similar copper–phen complexes, the two units are bonded via four Cu–O chemical bonds in compound 1; however, compound 2 is discreted and stabilized by intermolecular electrostatic interactions. Importantly, these Cu-POMs catalysts were first applied in the novel reaction for the preparation of 2-phenylquinoxalines via the one-pot coupling and oxidation reactions of 2-haloanilines with vinyl azides or 3-phenyl-2H-azirines under mild conditions, and Cu-POMs 1 showed higher catalytic performance in good yields (79–84%). The reactions exhibit some functional group tolerance and allow for the preparation of a number of 2-phenylquinoxalines.


 Please wait while we load your content...
Please wait while we load your content...