A new half-condensed Schiff base platform: structures and sensing of Zn2+ and H2PO4− ions in an aqueous medium†
Abstract
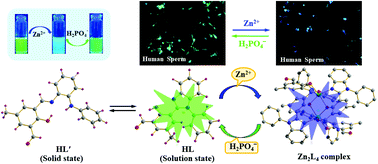
A newly designed and synthesized half-condensed organic moiety 2-hydroxy-5-methyl-3-[(2-phenylamino-phenylimino)-methyl]-benzaldehyde (HL′) and a Zn2L4 complex sequentially detect Zn2+ and H2PO4− ions as low as 1.13 nM and1.23 μM, respectively. HL′ and a dinuclear Zn(II) complex of in situ generated L− in a solution formulated as Zn2L4 under investigation were characterized by physicochemical and spectroscopic studies along with detailed structural analyses by single-crystal X-ray crystallography. The selectivity and sensitivity of HL′ towards Zn2+ ions and of the Zn2L4 complex towards H2PO4− ions are based on CHEF and via displacement pathways, respectively. Dual sensing of Zn2+ ions and H2PO4−ions in an aqueous medium via “Green-Blue-Green” emission with the reversible transformation of in situ formed HL′ to HL was established by detailed electronic absorption and emission spectroscopic studies. This non-cytotoxic probe (HL′, i.e. produced HL in solution) and Zn2L4 complexes are able to monitor the subcellular distribution changes of Zn2+ and H2PO4− ions, respectively, by fluorescence microscopy using the human semen sample.


 Please wait while we load your content...
Please wait while we load your content...