Copper(ii) and zinc(ii) complexes of mono- and bis-1,2,3-triazole-substituted heterocyclic ligands†
Abstract
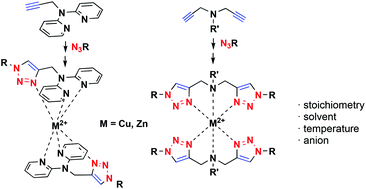
Chelating 1,4-disubstituted mono- (8a–8d) and bis-1,2,3-triazole-based (9a–11a) ligands were prepared by regioselective copper(I)-catalysed 1,3-dipolar cycloaddition of terminal alkynes with aromatic azides, together with bioconjugate 13a synthesized by amide coupling of L-phenylalanine methyl ester to 11a. Cu(II) and Zn(II) complexes were prepared and single crystal structures were determined for complexes 8aCu, 8dCu, 9cCu and 10cCu, as well as the free ligands 10a and 10c. The in situ prepared Zn(II) complexes were studied by NMR spectroscopy, while the stoichiometry of the Cu(II) complexes in solution was determined by UV-Vis titrations and confirmed by the electronic structure DFT calculations at the (SMD)/M05-2X/6-31+G(d)/LanL2DZ+ECP level of theory.


 Please wait while we load your content...
Please wait while we load your content...