Proton donor effects on the reactivity of SmI2. Experimental and theoretical studies on methanol solvation vs. aqueous solvation†
Abstract
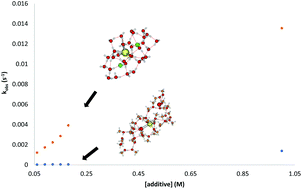
Proton donors are important components of many reactions mediated by samarium diiodide (SmI2). The addition of water to SmI2 creates a reagent system that enables the reduction of challenging substrates through proton-coupled electron-transfer (PCET). Simple alcohols such as methanol are often used successfully in reductions with SmI2 but often have reduced reactivity. The basis for the change in reactivity of SmI2–H2O and SmI2–MeOH is not apparent given the modest differences between water and methanol. A combination of Born–Oppenheimer molecular dynamics simulations and mechanistic experiments were performed to examine the differences between the reductants formed in situ for the SmI2–H2O and SmI2–MeOH systems. This work demonstrates that reduced coordination of MeOH to Sm(II) results in a complex that reduces arenes through a sequential electron proton transfer at low concentrations and that this process is significantly slower than reduction by SmI2–H2O.


 Please wait while we load your content...
Please wait while we load your content...