Nitrogen oxyanion reduction by Co(ii) augmented by a proton responsive ligand: recruiting multiple metals†
Abstract
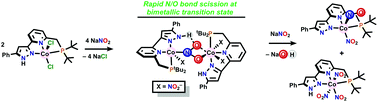
Deoxygenation of nitrite oxygen with divalent cobalt was achieved using (PNNH)CoCl2, carrying a pyridyl pincer ligand with one P(t-Bu)2 arm and one pyrazole arm. Reaction of (PNNH)CoCl2 with NaNO2 at a 2 : 5 mole ratio promptly forms equimolar (PNNH)Co(NO2)3 and (PNN)Co(NO2)(NO), {CoNO}8 with the lost ligand proton combined with removed oxo as hydroxide. These two CoIII products are characterized, showing a bent CoNO unit as the fate of the reduced nitrogen. DFT calculations are consistent with two one-electron CoII reductants binding to one NO2− bridge, then proton transfer being needed for facile N/O bond scission. A species detected by low temperature execution of this reaction contains cobalt in two oxidation states with an N,O bridging nitro group and pincer ligands that have been deprotonated, showing the active participation of the proton responsive ligand.


 Please wait while we load your content...
Please wait while we load your content...