Synthesis and characterisation of Co(iii) complexes of N-formyl hydroxylamines and antibacterial activity of a Co(iii) peptide deformylase inhibitor complex†
Abstract
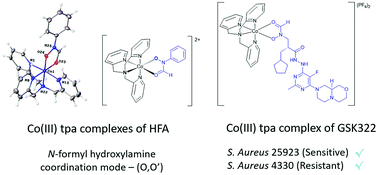
The X-ray crystal structure and pKa values of GSK322, a well-known and effective peptide deformylase inhibitor and antibacterial drug candidate, are reported. The first examples of Co(III) complexes of N-formyl hydroxylamines are reported. Reaction of N-hydroxy-N-phenylformamide (HFA) with [Co(tren)Cl2]Cl and [Co(tpa)Cl2]Cl (where tren = tris(2-aminoethyl)amine, tpa = tris(2-pyridylmethyl)amine) with one equivalent of NaOH in H2O afforded [Co(tren)(HFA−1H)](PF6)1.5Cl0.5 (1) and [Co(tpa)(HFA−1H)]Cl2 (2), respectively. X-ray crystal structures of both complexes revealed that the N-formyl hydroxylamine group acts as a bidentate ligand, coordinating the Co(III) centres via the carbonyl oxygen and deprotonated hydroxy group (O,O′), a coordination mode typically observed for closely related mono-deprotonated hydroxamic acids. Reaction of the N-formyl hydroxylamine-based GSK322 with [Co(tpa)Cl2]Cl afforded the corresponding Co(III) chaperone complex of the peptide deformylase inhibitor, [Co(tpa)(GSK322−1H)](PF6)2. GSK322 and [Co(tpa)(GSK322−1H)](PF6)2 exhibited better Gram-positive activity than Gram-negative, where low MICs (1.56–6.25 μM) were determined for S. aureus strains, independent of their antibiotic susceptibility.


 Please wait while we load your content...
Please wait while we load your content...