Phosphasalen group IV metal complexes: synthesis, characterization and ring opening polymerization of lactide†
Abstract
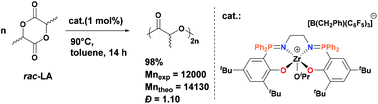
We report the synthesis of a series of Zr and Ti complexes bearing phosphasalen which differs from salen by the incorporation of two P atoms in the ligand backbone. The reaction of phosphasalen proligands (1a–1c)H2 with Zr(CH2Ph)4 led to different products depending on the nature of the N,N-linker in the ligand. In the case of ethylene-linked phosphasalen, octahedral Zr complex 2a formed as a single stereoisomer in trans geometry. With the phenylene linker, it was shown by dynamic NMR spectroscopy that complex 2b exists as a mixture of trans and cis-β isomers in solution, both enantiomers (Δ and Λ) of the cis-β isomer being in fast equilibrium with respect to the NMR time-scale. The use of a propylene-linked phosphasalen proligand 1cH2 led to a mixture of complexes among which a binuclear Zr complex 2c bridged only by one phosphasalen ligand could be isolated and characterized. Addition of 2 equiv. of iPrOH to 2a and 2b afforded diisoproxy Zr complexes 3a and 3b as a mixture of trans and cis-β isomers, the latter undergoing fast Δ/Λ isomerization in solution. Addition of B(C6F5)3 to 2a and 2b gave cationic monobenzyl Zr complexes 4a and 4b which have been further converted into cationic alkoxy Zr complexes 5a–b and 6a–b by alcoholysis with iPrOH and (S)-methyl-lactate, respectively. The reaction of the phosphasalen proligands with Ti(NMe2)4 proceeded diastereoselectively giving rise to Ti complexes 7a–c in octahedral geometry with cis-β wrapping of the ligand. The complexes have been tested for the ROP of rac-lactide. The neutral phosphasalen Ti and Zr complexes showed only poor activity probably due to the encumbered and electron donating nature of the phosphasalen ligand. In contrast, the cationic Zr alkoxides 5a, 6a and 6b are effective initiators for the controlled and hetero-selective ROP of rac-lactide.


 Please wait while we load your content...
Please wait while we load your content...