High-quality synthesis of a nanosized CHA zeolite by a combination of a starting FAU zeolite and aluminum sources†
Abstract
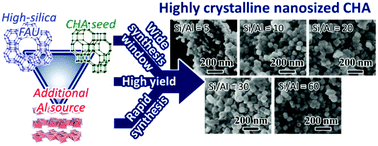
A chabazite (CHA zeolite) was synthesized using high-silica faujasite (FAU) zeolites with a Si/Al ratio of 93, an additional alumina source (aluminum hydroxide) combined with seed crystals, and N,N,N-trimethyl-1-adamantammonium hydroxide. We compared the crystallization behavior of the starting material (HSY + Al) with that of other combinations of silica/alumina sources (high-silica and low-silica FAU, fumed silica, and aluminum hydroxide). HSY + Al rapidly yielded nanosized CHA zeolites with a crystal size of approximately 70 nm, exhibited high product crystallinity and high yield and offered a wide synthesis window. A combination of analytic experiments using electrospray-ionization mass spectrometry and nuclear magnetic resonance (NMR) suggested that in the early stage, the pre-introduced CHA seeds provide a crystal nucleus and the FAU zeolites decompose to form oligomer species in the liquid phase. Meanwhile, aluminum hydroxide retains its solid phase. Subsequent crystallization of the zeolites is accelerated by the liquid silicate oligomer and solid aluminate sources, resulting in a high yield and rapid synthesis of nanosized CHA zeolites. We observed that phosphorus-modified CHA zeolites synthesized using HSY + Al perform well as a catalyst for ethanol conversion reactions. Controlled Si/Al ratios and additional phosphorus modifications improve catalytic durability, thereby exhibiting a higher propylene yield from the reaction within the zeolite pore system.


 Please wait while we load your content...
Please wait while we load your content...