Synthesis and group 9 complexes of macrocyclic PCP and POCOP pincer ligands†
Abstract
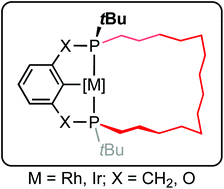
The synthesis of macrocyclic variants of commonly employed phosphine-based pincer (pro)ligands derived from meta-xylene (PCP-14) and resorcinol (POCOP-14) is described, where the P-donors are trans-substituted with a tetradecamethylene linker. The former was accomplished using a seven-step asymmetric procedure involving (−)-cis-1-amino-2-indanol as a chiral auxiliary and ring-closing olefin metathesis. A related, but non-diastereoselective route was employed for the latter, which consequently necessitated chromatographic separation from the cis-substituted by-product. The proligands are readily metalated and homologous series of MI(CO) and MIIICl2(CO) derivatives (M = Rh, Ir) have been isolated and fully characterised in solution and the solid state. Metal hydride complexes are generated during the synthesis of the former and have been characterised in situ using NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...