Synthesis and rhodium complexes of macrocyclic PNP and PONOP pincer ligands†
Abstract
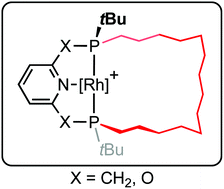
The synthesis of macrocyclic variants of commonly employed phosphine-based pincer ligands derived from lutidine (PNP-14) and 2,6-dihydroxypyridine (PONOP-14) is described, where the P-donors are trans-substituted with a tetradecamethylene linker. This was accomplished using an eight-step procedure involving borane protection, ring-closing olefin metathesis, chromatographic separation from the cis-substituted diastereomers, and borane deprotection. The rhodium coordination chemistry of these ligands has been explored, aided by the facile synthesis of 2,2′-biphenyl (biph) adducts [Rh(PNP-14)(biph)][BArF4] and [Rh(PONOP-14)(biph)][BArF4] (ArF = 3,5-(CF3)2C6H3). Subsequent hydrogenolysis enabled generation of dihydrogen, ethylene and carbonyl derivatives; notably the ν(CO) bands of the carbonyl complexes provide a means to compare the donor properties of the new pincer ligands with established acyclic congeners.


 Please wait while we load your content...
Please wait while we load your content...