A systematic investigation of structural transformation in a copper pyrazolato system: a case study†
Abstract
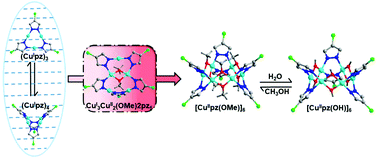
Dissolution-recrystallization structural transformation (DRST) is a powerful tool to unravel unequivocally the mechanism of dynamic conversion processes, based on the structures of the initial reactants, final products and sometimes intermediates isolated from the reaction mixture. Herein, we illustrate the details of the conversion processes of (CuIpz)3 into [CuII(OH)pz]6 (pzH = 4-chloro-3,5-diphenylpyrazole) through DRSTs. Based on crystal structure determination and spectroscopic methods, the most encountered species, (CuIpz)3, is in equilibrium with (CuIpz)4 in solution with the tetramer becoming dominant at low temperature, indicating an entropy-controlled conversion between these two structural isomers. The CuI trimer/tetramer in solution further experiences an oxidation if exposed to the open air, resulting in the formation of a pentanuclear mixed-valence intermediate CuI3CuII2(OMe)2pz5 which can be isolated as single crystals at −20 °C and has been structurally characterized for the first time. The final product isolated from the solution is the fully oxidized hexanuclear [CuII(OMe)pz]6, which is easily transformed into [CuII(OH)pz]6 in the presence of humidity.


 Please wait while we load your content...
Please wait while we load your content...