La3+:Ni–Cl oxyhydroxide gels with enhanced electroactivity as positive materials for hybrid supercapacitors†
Abstract
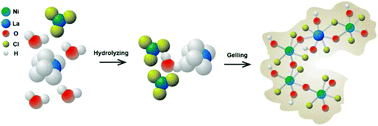
Electrochemical energy storage performance of inorganic solid materials is highly dependent on the number of electroactive sites and their reactivity. However, it is difficult to control the number of electroactive sites and their reactivity during the solid crystallization process. Herein, we synthesized novel poorly crystalline La3+:Ni–Cl oxyhydroxide gels with sufficient electroactive sites and atomically homogeneous distribution of Ni2+, La3+, and Cl− ions. Their poorly crystalline structure may favor fast ion diffusion and Cl− ions help in the formation of electroactive sites, while La3+ ions enhance the ionic conductivity within these electrode materials. Used as positive materials for hybrid supercapacitors, La3+:Ni–Cl oxyhydroxide gels showed a high specific capacity of 203 mA h g−1, a value higher than most reported values of Ni(OH)2-based materials. This work represents a new strategy for the synthesis of new electrode materials with high capacity and fast ion diffusion kinetics.


 Please wait while we load your content...
Please wait while we load your content...