Copper-based redox shuttles supported by preorganized tetradentate ligands for dye-sensitized solar cells†
Abstract
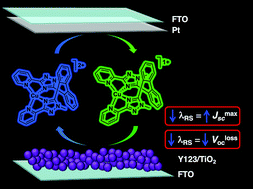
Three copper redox shuttles ([Cu(1)]2+/1+, [Cu(2)]2+/1+, and [Cu(3)]2+/1+) featuring tetradentate ligands were synthesized and evaluated computationally, electrochemically, and in dye-sensitized solar cell (DSC) devices using a benchmark organic dye, Y123. Neutral polyaromatic ligands with limited flexibility were targeted as a strategy to improve solar-to-electrical energy conversion by reducing voltage losses associated with redox shuttle electron transfer events. Inner-sphere electron transfer reorganization energies (λ) were computed quantum chemically and compared to the commonly used [Co(bpy)3]3+/2+ redox shuttle which has a reported λ value of 0.61 eV. The geometrically constrained biphenyl-based Cu redox shuttles investigated here have lower reorganization energies (0.34–0.53 eV) and thus can potentially operate with lower driving forces for dye regeneration (ΔGreg) in DSC devices when compared to [Co(bpy)3]3+/2+-based devices. The rigid tetradentate ligand design promotes more efficient electron transfer reactions leading to an improved JSC (14.1 mA cm−2), higher stability due to the chelate effect, and a decrease in VlossOC for one of the copper redox shuttle-based devices.


 Please wait while we load your content...
Please wait while we load your content...
