Deoxofluorination of graphite oxide with sulfur tetrafluoride†
Abstract
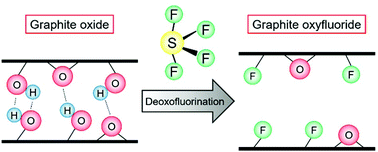
In this study, deoxofluorination of graphite oxide (GO) using sulfur tetrafluoride (SF4) at a temperature below the decomposition temperature of GO (∼200 °C) was investigated for the first time with and without HF catalysis. At 25 °C, the reaction proceeds only at high SF4 pressures (≥8 atm) when not catalyzed by HF and at 1 atm SF4 under the catalysis of HF. The degree of fluorination increases at higher temperatures and SF4 pressures. Hydroxy and carbonyl groups are replaced by fluorine following this reaction, and SF4 and SOF2 are introduced into the product, while the epoxy groups do not react. SF4 and SOF2 in the products are removed by washing with water. The obtained product is less hygroscopic than pristine GO owing to the hydrophobicity of the fluorine atom. The interlayer separation of the product is increased after deoxofluorination despite the smaller size of fluorine than the sizes of the oxygen-containing functional groups. When compared with direct fluorination using elemental fluorine, deoxofluorination using SF4 has the advantages of high reactivity with hydroxy groups and the preservation of the carbon skeleton, and the reaction results in the formation of graphite oxyfluoride.


 Please wait while we load your content...
Please wait while we load your content...