Kinetics mechanism insights into the oxygen evolution reaction on the (110) and (022) crystal facets of β-Cu2V2O7
Abstract
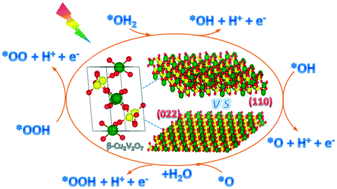
The sluggish kinetics for the oxygen evolution reaction (OER) seriously limits the photoelectrochemical (PEC) water oxidation performance of the monoclinic β-Cu2V2O7 which has been identified as a potential photoanode in a PEC cell. Among various strategies for improving the kinetics, tailoring the crystal facets of semiconductors is an effective one which has not been applied on β-Cu2V2O7 so far. We therefore study the OER kinetics mechanism on the (110) and (022) facets of β-Cu2V2O7 using the density functional theory (DFT). On both (110) and (022), the rate-limiting step is the dehydrogenation of the *OH group. The adsorbates (O and OH species) adjacent to the reaction sites are able to facilitate this elementary step. With and without the neighboring adsorbates, *OH dehydrogenation is more kinetically favorable on the (110) facet compared to the (022) surface. In addition, the dissociation of *OOH to form *OO is spontaneous and barrierless in all cases. The product O2 is easier to desorb from the (110) facet than the (022) surface. Our results suggest that the (110) orientation is more active than (022) for the OER, which will be helpful for tailoring the crystal facets of β-Cu2V2O7 in experiments.


 Please wait while we load your content...
Please wait while we load your content...