The role of photocatalysts in radical chains in homolytic aromatic substitution, radical addition to olefins, and nucleophilic radical substitution mechanisms†
Abstract
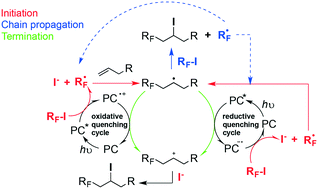
Photoinitiated perfluorobutylation reactions of 2-anisidine 1, propenyloxybenzene 2, and 2-mercaptoethanol 3 have been carried out employing commercially available n-C4F9I as the source of ˙C4F9 radicals under three different photocatalysts (PCs) absorbing in the violet (Ir[dF(CF3)ppy]2(dtbbpy)+ (PC-1)), green (Rose Bengal (PC-2)) and red (zinc phthalocyanine PhZn (PC-3)) regions of the electromagnetic spectrum, in order to explore the quantum yields and radical chain lengths for each type of transformation and photoinitiated system. For substrates 1–3 and their different types of reactions, the comparative study between PCs seeks to establish the extent to which regioproducts, yields, and mechanistic proposals are influenced by the intervention of each PC. The different overall quantum yields and chain lengths obtained for each type of reaction under each PC are a direct consequence of the efficiency of the photoinitiation events, i.e., primary radical production, propagation and termination steps. This will have important implications in the understanding and representations of the mechanisms postulated, in terms of closed and open catalytic cycles. One strategy we apply to uncover radical propagating chains in our mechanisms is to explore photocatalysts that operate in oxidative and reductive quenching manners for each type of transformation.


 Please wait while we load your content...
Please wait while we load your content...