Does the structure of CuZn hydroxycarbonate precursors affect the intrinsic hydrogenolysis activity of CuZn catalysts?†
Abstract
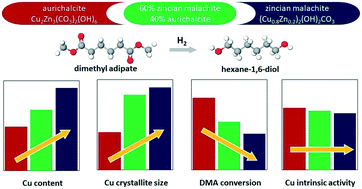
A comparative study of the properties and hydrogenolysis activity of catalysts derived from single-phase CuZn precursors was carried out to elucidate the effect of the precursor structure on the catalyst activity in hydrogenolysis and to examine their potential to replace the classical CuCr catalysts. Three CuZn catalysts were prepared by co-precipitation aiming at Cu/Zn ratios of 0.7, 5.0 and 1.6 to obtain single-phase aurichalcite, zincian malachite and a mixture of both phases, respectively. The catalyst precursors were calcined at temperatures of 300 and 450 °C, reduced in situ and tested in dimethyl adipate hydrogenolysis. The properties of the precursors and catalysts were analyzed using in situ XRD, TGA-MS, TPR-H2, nitrogen physisorption and N2O chemisorption methods. According to the characterization data, aurichalcite-derived catalysts had superior structural properties to zincian malachite-derived catalysts: smaller copper crystallites, larger total and copper surface areas, and stable surface areas during the experiment. Consequently, aurichalcite-derived catalysts outperformed catalysts prepared from zincian malachite in the studied range of phase and elemental CuZn compositions. Nonetheless, the calculated intrinsic hydrogenolysis activity of surface Cu sites (TOF) was similar for all three catalysts.


 Please wait while we load your content...
Please wait while we load your content...