Photocatalytic asymmetric epoxidation of trans-stilbene with manganese–porphyrin/graphene-oxide nanocomposite and molecular oxygen: axial ligand effect†
Abstract
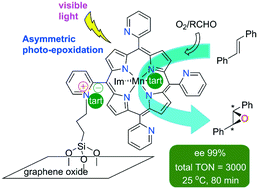
An efficient, visible light-driven manganese–porphyrin photocatalyst was developed for the asymmetric epoxidation by molecular oxygen under mild conditions. A Mn–porphyrin complex covalently bonded to graphene oxide (GO) sheets was synthesized and characterized, where chirality is induced by enantiopure L-tartrate (tart), acting either as a counter ion or axial ligand. The heterogeneous photocatalyst GO-[Mn(T2PyP)(tart)](tart) showed an excellent epoxide selectivity of 100% toward the enantioselective epoxidation of trans-stilbene (ee 100%) in the presence of imidazole under irradiation with a white LED light source. An imidazole molecule hydrogen bonded to the high-valent manganese–oxo intermediate and (tartrate)− counter ion seems to be responsible for substantially enhancing the enantioselectivity of the catalyst. Also, an imidazole molecule coordinated to the metal centre is probably involved in the increased catalytic activity. With immobilized manganese porphyrin as photocatalyst, significant improvements in rate and enantioselectivity were attained by simply adding imidazole as an axial ligand and a hydrogen bond donor in trans-stilbene epoxidation. At the end of the reaction, GO-[Mn(T2PyP)(tart)](tart) was readily separated by filtration and reused for subsequent runs without any loss of its activity and enantioselectivity, resulting in a total turnover number (TON) of 3000.


 Please wait while we load your content...
Please wait while we load your content...