Ir–Biaryl phosphite–oxazoline catalyst libraries: a breakthrough in the asymmetric hydrogenation of challenging olefins
Abstract
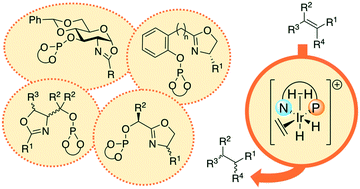
Asymmetric hydrogenation (AH) is one of the most attractive methods for the preparation of enantiomerically pure compounds due to its operational simplicity and perfect atom economy. Despite the extensive research dedicated to the AH of alkenes and the important progress achieved, some issues still need to be solved. Most catalysts only work with a limited number of alkenes and each type of alkene needs a specific catalyst for optimal enantioselectivity. In this respect, the AH of functionalized alkenes is mostly carried out with Ru– and Rh–diphosphine catalysts, while the AH of olefins without a coordinative functional group is mainly carried out with Ir–P,N catalysts. The AH of functionalized olefins has been thoroughly studied for decades; there are, however, some substrate types that are still a challenge (e.g. cyclic β-enamides). Compared to the AH of functionalized olefins, the reduction of unfunctionalized alkenes is much less developed. Most catalysts are still specific for the type of olefin geometry and its substitution pattern. Most successful cases have been reported for trisubstituted E-unfunctionalized alkenes and, to a lesser extent, for Z-trisubstituted, 1,1′-disubstituted and tetrasubstituted olefins. Broad substrate scopes are desirable to reduce the time dedicated to ligand design and preparation. Our group contributed in this field with a series of improved ligand libraries that considerably expanded the substrate scope. In this minireview, we reveal our progress in the iridium-catalyzed AH of a broad range of unfunctionalized olefins and cyclic β-enamides.


 Please wait while we load your content...
Please wait while we load your content...