Catalysis, kinetics and mechanisms of organo-iridium enantioselective hydrogenation-reduction
Abstract
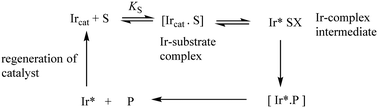
The synthesis of chiral molecules is of great importance to the pharmaceutical, agrochemical, flavour and fragrance industries. The use of organo-iridium complexes has gained a reputation for its great utility in key enantioselective synthetic procedures. Prime examples include the catalytic reduction of carbonyls and imines; iridium-catalysed allylic substitution and catalysed enantioselective hydrogenation of unsaturated carboxylic acids. Important aspects in these processes are the reaction conditions such as the catalyst loading, metal-ion ligands, the substrate, solvent and the reaction times-all of which can affect the degree of enantioselectivity. Understanding the mechanisms of these hydrogenation/reduction reactions through kinetic and other related studies makes a vital contribution to improving catalytic efficiency.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...