The ditetrel bond: noncovalent bond between neutral tetrel atoms
Abstract
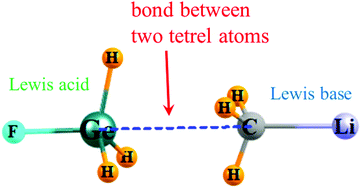
The ability of a tetrel atom to serve in the capacity of electron donor in a σ-hole noncovalent bond is tested by quantum calculations. The donor molecule TMH3 contains a metal atom M on a tetravalent tetrel (T) atom, with M = Li, Na, K so as to generate a partial negative charged region on T. The F atom of the Lewis acid H3FT molecule facilitates the formation of a positive σ-hole on the acid T atom. The CLiH3 Lewis base engages in the strongest T⋯T tetrel bonds (TBs), with interaction energies as high as 10 kcal mol−1. The larger T atoms, whether Si, Ge, or Sn, form weaker TBs, with little dependence on their identity within this subgroup. In contrast, there is a strong dependence of the interaction energy on the size of the acidic tetrel atom, growing quickly in the order C < Si < Ge < Sn < Pb. The interaction energies correlate closely with (i) the total charge transferred, (ii) the stretch of the covalent T–F bond and (iii) the product of the extrema on the electrostatic potentials. The charge being transferred originates in the four covalent bonds of the Lewis base molecule. The systems bear striking resemblance to dihydrogen bonds where two H atoms of opposite charge attract one another.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...