Electrochemical stability of glyme-based electrolytes for Li–O2 batteries studied by in situ infrared spectroscopy†
Abstract
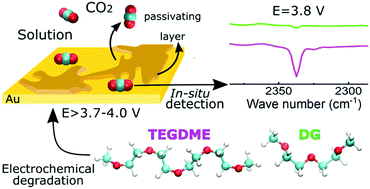
In situ subtractively normalized Fourier transform infrared spectroscopy (SNIFTIRS) experiments were performed simultaneously with electrochemical experiments relevant to Li–air battery operation on gold electrodes in two glyme-based electrolytes: diglyme (DG) and tetraglyme (TEGDME), tested under different operational conditions. The results show that TEGDME is intrinsically unstable and decomposes at potentials between 3.6 and 3.9 V vs. Li+/Li even in the absence of oxygen and lithium ions, while DG shows a better stability, and only decomposes at 4.0 V vs. Li+/Li in the presence of oxygen. The addition of water to the DG based electrolyte exacerbates its decomposition, probably due to the promotion of singlet oxygen formation.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...