Symmetry breaking of the bending mode of CO2 in the presence of Ar†
Abstract
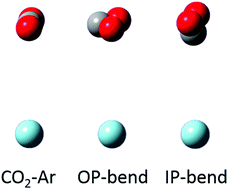
The weak infrared spectrum of CO2–Ar corresponding to the (0111) ← (0110) hot band of CO2 is detected in the region of the carbon dioxide ν3 fundamental vibration (≈2340 cm−1), using a tunable OPO laser source to probe a pulsed supersonic slit jet expansion. While this method was previously thought to cool clusters to the lowest rotational states of the ground vibrational state, here we show that under suitable jet expansion conditions, sufficient population remains in the first excited bending mode of CO2 (1–2%) to enable observation of vibrationally hot CO2–Ar, and thus to investigate the symmetry breaking of the intramolecular bending mode of CO2 in the presence of Ar. The bending mode of the CO2 monomer splits into an in-plane and an out-of-plane mode, strongly linked by a Coriolis interaction. Analysis of the spectrum yields a direct measurement of the in-plane/out-of-plane splitting measured to be 0.8770 cm−1. Calculations were carried out to determine if key features of our results, i.e., the sign and magnitude of the shift in the energy for the two intramolecular bending modes, are consistent with a quantum chemical potential energy surface. This aspect of intramolecular interactions has received little previous experimental and theoretical consideration. Therefore, we provide an additional avenue by which to study the intramolecular dynamics of this simplest dimer in its bending modes. Similar results should be possible for other weakly-bound complexes.


 Please wait while we load your content...
Please wait while we load your content...