Solvent oligomerization pathways facilitated by electrolyte additives during solid-electrolyte interphase formation†
Abstract
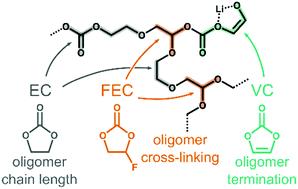
The solid–electrolyte interphase (SEI) layer formation is known to play an important role in determining the lifetime of lithium-ion batteries. A thin, stable SEI layer is linked to overall improved battery performance and longevity, however, the factors and mechanisms that lead to optimal SEI morphology and composition are not well understood. Inclusion of electrolyte additives (fluoroethylene carbonate, FEC; and vinylene carbonate, VC) is often necessary for improving SEI characteristics. To understand how these electrolyte additives impact SEI formation, molecular dynamics (MD) and density functional theory (DFT) simulations were employed to study the reaction networks and oligomerization pathways, respectively, for three systems containing ethylene carbonate (EC), a lithium ion, and FEC or VC. MD simulations suggest radical oligomerization pathways analogous to traditional oligomerization with nucleophilic alkoxide species via SN1 reaction mechanisms. Both SN1 and SN2 mechanisms were studied for all three systems using DFT. Oligomerization reactions were studied with both a standard alkoxide species and a ring-opened EC radical as the nucleophiles and EC, FEC, and VC as the electrophiles. For all cases, FEC and VC exhibited lower free energy barriers and more stable adducts when compared with EC. We conclude that one of the role of additives is to modify the oligomerization process of EC by introducing branching points (FEC) or termination points (VC).


 Please wait while we load your content...
Please wait while we load your content...