Hydrate formation and its effects on the thermal expansion properties of HfMgW3O12†
Abstract
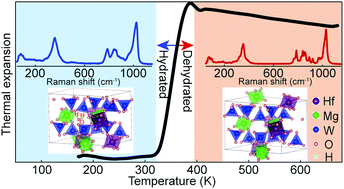
HfMgW3O12 is a representative material with negative thermal expansion in the ABM3O12 (A = Zr, Hf; B = Mg, Mn, Zn, M = W, Mo) family. Herein we report a novel feature of hydration in HfMgW3O12 and its effect on the thermal expansion and its structures which have not been determined previously. It is found that hydrate formation in HfMgW3O12 occurs under ambient or moisture conditions and restrain the low energy librational and translational and even high energy bending and stretching motions of the polyhedra. The coefficient of thermal expansion could be tailored from negative to zero and positive depending on the hydration levels. The unhydrated HfMgW3O12 adopts an orthorhombic structure with space group Pna21 (33) without phase transition at least from 80 K to 573 K, but pressure-induced structure transition and amorphization are found to occur at about 0.19 Gpa and above 3.93 GPa, respectively.


 Please wait while we load your content...
Please wait while we load your content...