Unconventional stable stoichiometry of vanadium peroxide†
Abstract
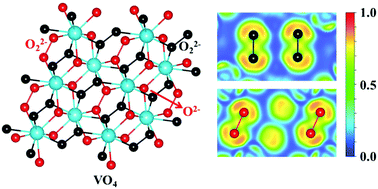
Peroxides have attracted considerable attention due to their intriguing electronic properties and diverse applications. However, only a few transition metal peroxides have been known thus far, limiting the variety of peroxide examples. Here, we demonstrate the stabilization of peroxides in the O-rich V–O system through first-principles calculations coupled with a swarm-intelligence structure search. As well as reproducing the known stoichiometries of VO, V2O3, VO2, and V2O5, two hitherto unknown V2O and VO4 stoichiometries are predicted to be thermodynamically stable at megabar pressures. VO4 has the highest oxygen content among the known peroxides to date. More interestingly, its electronic band gap increases with pressure, originating from the pressure-induced decrease of O–O bonding length in the peroxide group. V-rich V2O exhibits superconductivity, becoming the first example in the V–O system. Our work not only unravels the unusual vanadium peroxide, but also provides further insight into the diverse electronic properties of vanadium oxides under high pressure.


 Please wait while we load your content...
Please wait while we load your content...