Elucidation of the role of guanidinium incorporation in single-crystalline MAPbI3 perovskite on ion migration and activation energy†
Abstract
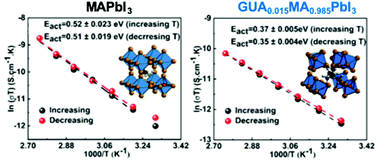
Ion migration plays a significant role in the overall stability and power conversion efficiency of perovskite solar cells (PSCs). This process was found to be influenced by the compositional engineering of the A-site cation in the perovskite crystal structure. However, the effect of partial A-site cation substitution in a methylammonium lead iodide (MAPbI3) perovskite on the ion migration process and its activation energy is not fully understood. Here we study the effect of a guanidinium (GUA) cation on the ion transport dynamics in the single crystalline GUAxMA1−xPbI3 perovskite composition using temperature-dependent electrochemical impedance spectroscopy (EIS). We find that the small substitution of MA with GUA decreases the activation energy for iodide ion migration in comparison to pristine MAPbI3. The presence of a large GUA cation in the 3D perovskite structure induces lattice enlargement, which perturbs the atomic interactions within the perovskite lattice. Consequently, the GUAxMA1−xPbI3 crystal exhibits a higher degree of hysteresis during current–voltage (J–V) measurements than the single-crystalline MAPbI3 counterpart. Our results provide the fundamental understanding of hysteresis, which is commonly observed in GUA-based PSCs and a general protocol for in-depth electrical characterization of perovskite single crystals.


 Please wait while we load your content...
Please wait while we load your content...