Effect of substituents on surface equilibria of thiophenols and isoquinolines on gold substrates studied using surface-enhanced Raman spectroscopy
Abstract
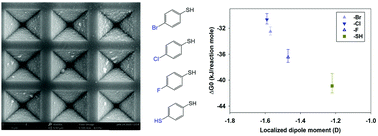
The effect of substituents on the surface adsorption equilibria of thiophenols and isoquinolines on gold substrates was studied using surface-enhanced Raman spectroscopy (SERS) in order to determine the effects of the localized dipole moments and charge donating/withdrawing properties on the binding affinity. Two common classes of molecules used in SERS studies were examined, which included substituted aromatic thiols and nitrogen heterocyclic aromatic molecules (azaarenes), due to their strong affinity for gold surfaces. Unsubstituted thiophenol in aqueous solution binds strongly to gold surfaces. Therefore, it is difficult to measure an equilibrium constant, since even at concentrations of 10−8 M nearly a complete self-assembled monolayer (SAM) forms. In contrast, substituted thiophenols with electron-withdrawing groups, such as halogenated thiophenols, bind much less strongly, allowing equilibrium constants to be obtained. It is believed that the substituent withdrawing charge away from the sulfur atom affects the adsorption/binding between the analyte and surface. Thiophenols substituted with electron donating groups behaved similar to unsubstituted thiophenol, where a SAM was observed at concentrations as low as 10−8 M. These functional groups did not hinder the ability of the sulfur groups to bind with gold. In addition, a series of bromine-substituted isoquinolines, a group of azaarene compounds, were measured to determine the effects that the bromine substituent has when it is bound to the two different rings and if position on the rings has an effect. The azaarene class of molecules, including isoquinoline, adsorbs less strongly than thiophenols, and a dual Langmuir isotherm phenomenon is observed where protonated and neutral bromoisoquinoline molecules occupy two different types of sites on Klarite substrates, which consist of inverted micro-pyramids on Si wafers with rough/nanostructured Au coatings. Protonated isoquinolines bind to nucleophilic sites on the substrates which tend to occur on flatter regions of the substrate. By contrast, neutral isoquinolines bind to electrophilic sites which are predominant near microscopic edges on the substrate. The presence of the bromine substituent and its position in the fused ring structure changes the Gibbs free energies of adsorption, depending on which ring the substituent is in. These results can help to guide the development of SERS for analytical applications by demonstrating how changes in functional groups can affect the equilibrium constants, which are critical for determining the effectiveness of SERS as a tool for trace detection of analytes.


 Please wait while we load your content...
Please wait while we load your content...