Hydrogen bonds and halogen bonds in complexes of carbones L→C←L as electron donors to HF and ClF, for L = CO, N2, HNC, PH3, and SH2†
Abstract
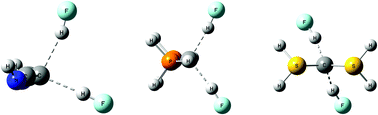
Ab initio MP2/aug’-cc-pVTZ calculations have been carried out to determine the structures and binding energies of the carbone complexes in which the carbone L→C←L acts as an electron pair donor to one and two HF or ClF molecules, for L = CO, N2, HNC, PH3, and SH2. The binding energies increase with respect to the ligand in the order CO < NN < CNH ≪ PH3 < SH2, and increase with respect to the acid in the order HF < 2 HF < ClF < 2 ClF. The complexes with the ligands CO, N2 and PH3 have C2v symmetry while those with CNH and SH2 have Cs symmetry, except for H2S→C←SH2:2HF which has C2 symmetry and a unique structure among all of the carbone complexes. F–H and Cl–F stretching frequencies in the complexes decrease as the F–H and Cl–F distances, respectively, increase. EOM-CCSD spin–spin coupling constants 2hJ(F–C) increase with decreasing F–C distance. Although the F–H⋯C hydrogen bonds gain some proton-shared character in the most tightly bound complexes, the hydrogen bonds remain traditional hydrogen bonds. 1xJ(Cl–C) values indicate that the Cl⋯C halogen bonds have chlorine shared character even at the longest distances. 1xJ(Cl–C) then increases as the Cl–C distance decreases, and reaches a maximum for chlorine-shared halogen bonds. As the Cl–C distance further decreases, the halogen bond becomes a chlorine-transferred halogen bond.


 Please wait while we load your content...
Please wait while we load your content...