Prediction of new thermodynamically stable ZnN2O3 at high pressure†
Abstract
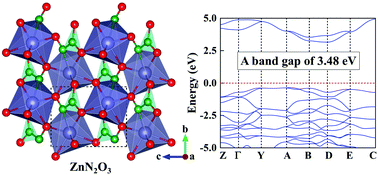
Pressure has become a useful parameter to prepare novel functional materials. Considering the excellent performance of ZnO and Zn3N2 and the formation of strong Zn–O, Zn–N, and N–O bonds in the known compounds, we explored potential Zn–N–O ternary compounds with interesting properties. With the aid of first-principles swarm-intelligence search calculations, we identified a hitherto unknown ZnN2O3 ternary compound with a symmetry of P21. Its remarkable feature is that N pairs interconnect the distorted Zn-centered decahedrons, in which the Zn atom forms bonds with one N and six O atoms. The compression of ZnO + NO2 + N2 might be an easy way to synthesize ZnN2O3. Electronic property calculations disclose that ZnN2O3 is a wide band gap semiconductor with a gap value of 3.48 eV, which is larger than those of ZnO and Zn3N2. Moreover, the high-pressure phase diagram of Zn–N binary compounds was explored with a wide range of chemical compositions. Two metallic N-rich zinc nitrides (e.g., ZnN2 and ZnN4) are proposed, containing intriguing N2 dimers and zigzag N chains. ZnN2 exhibits superconducting properties, and becomes the first example of superconductor in zinc nitrides. Our current results unravel the unusual stoichiometry of Zn–N–O compounds and provide further insight into the diverse electronic properties of zinc nitrides under high pressure.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...