Active stereo-control of the Cl + CH4(ν3 = 1) reaction: a three-dimensional perspective†
Abstract
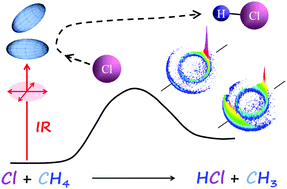
The transition state in Cl + CH4 is of Cl–H–C collinear geometry. As the reactant CH4 is vibrationally excited by a linearly polarized infrared (IR) light to the antisymmetric-stretching state of ν3 = 1, all four C–H bonds are collectively excited and any one of the H-atoms can be reactive. Yet, a strong alignment of the excited CH4(ν3 = 1), as evidenced from the striking stereo-specificity in the Cl + CH4 reaction, was clearly revealed in a previous, exploratory study. Reported here is the full account of that investigation at two collisional energies of Ec = 4.8 and 2.7 kcal mol−1, using a crossed molecular-beam, product-imaging approach. By active control of the polarization direction of an IR laser under judiciously chosen beam-geometries, a complete set of polarization-dependent differential cross sections is disentangled from the CH3(00) product images. To our surprise, the quantitative results appear nearly identical to those obtained for the isotope-substituted reaction of Cl + CHD3(ν1 = 1) → HCl(ν) + CD3(00). A detailed discussion is presented to elucidate the underlying physics for such an intriguing similarity in stereo-reactivity between a spherical-top and a symmetric-top reactant.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...