Computational insight into the halogen bonded self-assembly of hexa-coordinated metalloporphyrins†
Abstract
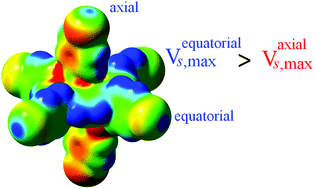
We demonstrate herein a computational study probing the influence of metalloporphyrins on intermolecular halogen bonding (XB) during supramolecular self-assembly. The results demonstrate that porphyrin aromatic rings can activate or deactivate halogen bonding interactions, especially those on axial ligands, and further influence the preference type of halogen···halogen bonding during the supramolecular self-assembly. Calculations show that the halogen atom present at the equatorial position has a higher sigma hole potential (VS,max) than that at the axial position. The computational analysis and our observations from the X-ray structure analysis are in good agreement. From structural analysis it is clear that equatorial halogen atoms prefer to participate in Type-II XB interactions whereas the axial halogen atoms either participate in Type-I XB interaction or reluctant to participate in XB interactions due to the decrease of their sigma hole potential. Thus, we demonstrate, herein, for the first time a computational study probing the direct influence of the porphyrin's ring current on the sigma hole potential (VS,max) of the halogen atoms and subsequently the effects of the supramolecular self-assembly.


 Please wait while we load your content...
Please wait while we load your content...