Simple photoreduction of carbon dioxide to formic acid and true quantum yield†
Abstract
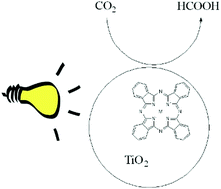
There is a need to develop techniques for conversion of carbon dioxide to useful products such as formaldehyde, formic acid, methanol, and hydrocarbons. Carbon dioxide can be converted into these products using either photochemically, electrochemically, thermochemical or hydrogenation by bacteria. Formate is of interest due to the possibility of being used in liquid fuel cells, as an additive in pyrolysis vapors and as a precursor for biological fuels. In this work, conversion of carbon dioxide to formic acid under acidic conditions and formate under basic or neutral conditions was accomplished through photoreduction using an inexpensive setup consisting of titanium dioxide, metal phthalocyanines and inexpensive incandescent sources. The yield of formic acid based on anion chromatography was 1.54%. This work also discusses and presents a true quantum yield determined using chemical actinometry which was near 2.0%. Detailed studies of the photoreduction process showed that the amount of sensitizer, light intensity and pH affect the amount of formate generated.


 Please wait while we load your content...
Please wait while we load your content...