What is the preferred geometry of sulfur–disulfide interactions?†
Abstract
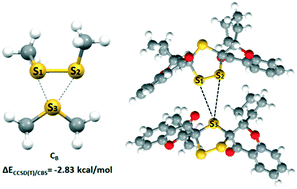
Non-covalent interactions between disulfide fragments and sulfur atoms were studied in crystal structures of small molecules and by quantum chemical calculations. Statistical analysis of the geometrical data from the Cambridge Structural Database (CSD) reveals that in most cases, interactions between sulfur and disulfide bonds are bifurcated. Quantum chemical calculations are in agreement with those findings. A strong interaction energy was calculated for bifurcated interactions (ECCSD(T)/CBS = −2.83 kcal mol−1) considering the region along the disulfide bond. Non-bifurcated interactions are weaker except in cases where σ-hole interaction is possible or in cases where S⋯S interaction is accompanied by additional hydrogen bonds (ECCSD(T)/CBS = −3.26 kcal mol−1). SAPT decomposition analysis shows that dispersion is the main attractive force in the studied systems while electrostatics plays a crucial role in defining the geometry of interactions.
- This article is part of the themed collection: The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...