Chirality-dependent supramolecular synthons based on the 1,3-oxazolidin-2-one framework: chiral drugs mephenoxalone, metaxalone and 114 other examples†
Abstract
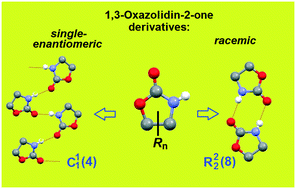
In four of the five crystalline modifications of the muscle relaxants mephenoxalone 3 and metaxalone 2, chain supramolecular motifs of three different types are realized. The centrosymmetric cyclic dimer, which is considered typical of amides, was found only once. To clarify the nature of the emerging supramolecular synthons, the set of 119 crystal structures of 1,3-oxazolidin-2-one 1 derivatives, to which drugs 2 and 3 belong, were selected from the Cambridge Structural Database. Analysis of the sample showed that oxazolidinone fragments predominantly form closed ring synthons in racemic crystals, whereas linear chains are typical for enantiopure ones. Thus, in the case of chiral objects, the transition from racemic to enantiopure crystal serves as a powerful tool for designing crystals with a given organization of supramolecular synthon. Two earlier unidentified kryptoracemates (false conglomerates), QEFJAP and QEFJUJ, were found among the analyzed set. Apparently, these are the first representatives of oxazolidinones exhibiting this rare property.
- This article is part of the themed collection: The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...