Catalytic enantioselective domino Michael/transannular aldol reaction under bifunctional catalysis†
Abstract
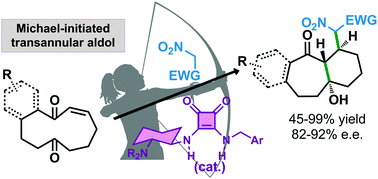
The enantioselective Michael reaction catalyzed by a bifunctional tertiary amine/squaramide has been used to trigger a Michael/transannular aldol cascade process that leads to densely substituted bicyclo[5.4.0]undecanes and in which three contiguous stereogenic centres, one of them a tertiary alcohol moiety, have been formed in a fully stereocontrolled fashion.


 Please wait while we load your content...
Please wait while we load your content...