Synthesis of Weinreb amides using diboronic acid anhydride-catalyzed dehydrative amidation of carboxylic acids†
Abstract
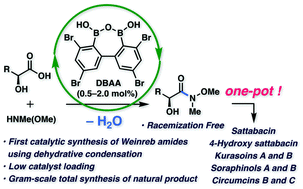
The first successful example of the direct synthesis of Weinreb amides using catalytic hydroxy-directed dehydrative amidation of carboxylic acids using the diboronic acid anhydride catalyst is described. The methodology is applicable to the concise syntheses of eight α-hydroxyketone natural products, namely, sattabacin, 4-hydroxy sattabacin, kurasoins A and B, soraphinols A and B, and circumcins B and C.


 Please wait while we load your content...
Please wait while we load your content...