Kinetic versus thermodynamic metalation enables synthesis of isostructural homo- and heterometallic trinuclear clusters†
Abstract
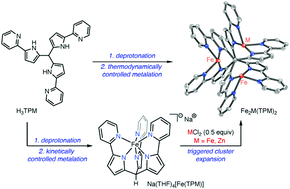
Temperature-dependent metalation of the new hexadentate ligand (tris(5-(pyridin-2-yl)-1H-pyrrol-2-yl)methane; H3TPM) enables the selective synthesis of both mononuclear (i.e. Na(THF)4[Fe(TPM)], kinetic product) and trinuclear (i.e. Fe3(TPM)2, thermodynamic product) complexes. Exposure of Na(THF)4[Fe(TPM)] to FeCl2 or ZnCl2 triggers cluster expansion to generate homo- or heterometallic trinuclear complexes, respectively. The developed approach enables systematic variation of ion content in isostructural metal clusters via programmed assembly.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...