Organocatalytic asymmetric synthesis of β,β-diaryl ketones via one-pot tandem dehydration/1,6-addition/decarboxylation transformation of β-keto acids and 4-hydroxybenzyl alcohols†
Abstract
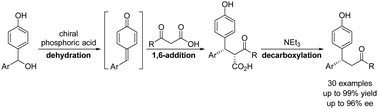
Herein we describe an organocatalytic protocol for the asymmetric synthesis of β,β-diaryl ketones. Under the catalysis of a chiral phosphoric acid, 4-hydroxybenzyl alcohols underwent dehydration to form para-quinone methides, which reacted with β-keto acids in 1,6-addition reactions. Upon treatment with Et3N in one-pot, decarboxylation proceeded to provide the desired chiral ketones in nearly quantitative yields with high enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...