Synthesis of tertiary amines by direct Brønsted acid catalyzed reductive amination†
Abstract
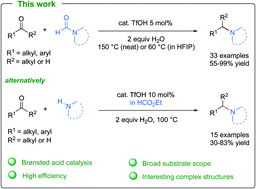
Tertiary amines are ubiquitous and valuable compounds in synthetic chemistry, with a wide range of applications in organocatalysis, organometallic complexes, biological processes and pharmaceutical chemistry. One of the most frequently used pathways to synthesize tertiary amines is the reductive amination reaction of carbonyl compounds. Despite developments of numerous new reductive amination methods in the past few decades, this reaction generally requires non-atom-economic processes with harsh conditions and toxic transition-metal catalysts. Herein, we report simple yet practical protocols using triflic acid as a catalyst to efficiently promote the direct reductive amination reactions of carbonyl compounds on a broad range of substrates. Applications of this new method to generate valuable heterocyclic frameworks and polyamines are also included.


 Please wait while we load your content...
Please wait while we load your content...